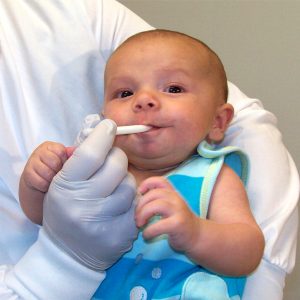
Studies have reported that some older preschoolers are able to donate saliva samples by spitting or drooling (1,2), but researchers have generally found that using some sort of absorbent device is the best way to collect saliva from young children. Parents often have a better chance of getting the child to accept the device into his or her mouth, but even they may not succeed if the child is frightened, cranky, or overexcited.
Safety is an important issue when choosing a collection device to use with children, due to the potential for choking. For this reason, we advise that our own SalivaBio Oral Swab (SOS) – a 1 x 3 cm swab made of an inert polymer – should be used only for adults and children 6 years and older.
Salimetrics previously recommended two devices that could be held by a parent or technician to insure that they were not choking hazards:
- Braided cotton dental rope. Although widely used in many studies for both children and adults (3-6), cotton was a poor collection material due to its unpleasant taste and texture, the difficulty of recovering the saliva and/or analytes from the cotton, and the fact that it causes interference with certain biomarkers, including testosterone, SIgA, estradiol, DHEA, and progesterone (7,8).
- The Sorbette – a small, arrowhead-shaped hydrocellulose sponge attached to a plastic shaft. (BD Opthalmic Systems Visipear, prod. no. 581089) The Sorbette was an improvement over cotton rope, with better overall recovery of volume and cortisol compared to cotton (7) and it has been successfully used in studies with young children (8-11). However, the volume recovered from the Sorbette could still be unacceptably low (7). This was mainly due to the sorbette appearing to have collected a sufficient sample volume, before reaching capacity, and because the capcity was limited to 200-300 µL per device. This led to researchers utilizing multiple sorbettes at the same time, and even then still failing to obtain enough sample to complete testing. An additional negative point was that Salimetrics approved the Sorbette for use only when testing for cortisol, α-amylase, cotinine, and SIgA, since it caused assay interference for other analytes. Similar “eyespear” devices from other manufacturers have also been found to cause varying degrees of assay interference for cortisol determination, unlike the Sorbette, and they should not be used for saliva collection without appropriate validation (12). Use of the Sorbette was also problematic when samples were to be tested for analytes that require expression of assay results in relation to saliva flow, such as SIgA and sAA. (13,14) Given its limited absorbent capacity, there was a real likelihood that the Sorbette would reach saturation very quickly when adequate saliva was present in the mouth. We therefore felt that it was probably not possible to reliably estimate flow rates by noting the length of time it was in the mouth and weighing the device to determine the volume absorbed. (14)
Given the need for a child-safe collection device that works with a broader range of analytes and that collects a larger volume, Salimetrics has introduced two alternative versions of the SOS: the SalivaBio Children’s Swab (SCS) (Item No. 5001.06), and the SalivaBio Infant’s Swab (SIS) (Item No. 5001.08) These devices are manufactured in longer lengths, which allows one end to be held by an adult while the other end is placed in the child’s mouth. The diameters of the SCS and SIS are appropriate for the size of the children’s mouths, 8 mm and 5 mm, respectively. The polymer used for the swabs is very durable and can withstand chewing by the child, and its taste and texture are also acceptable to children. Like the original SOS, samples collected with either the SCS or the SIS may be tested for multiple analytes. And, importantly, even at small collected volumes of 25-50 µL, the recoveries of saliva volume and cortisol from the SCS are = 90 and 95 %, respectively.
Either swab can also be centrifuged or compressed (in a 5 cc needless syringe) if desired. In this case, the wet end of the swab should be inserted into the storage tube (or syringe), followed by doubling the dry end over into the opening, and finally using the cap (or plunger) to push the entire swab into the interior space. This procedure will allow the entire swab and storage tube to be weighed before and after collection of samples for determination of SIgA or sAA, in order to estimate the saliva flow rate during the timed collection period. The issue of the swab remaining in the mouth after the point of saturation is still a concern, however, and estimates of the flow rate and secretion rate will be inaccurate if the collection period is too long (14). We therefore advise that some preliminary study should be done to determine the optimum collection duration for the type of subjects in the study.
Researchers with infants achieved the highest success rate for saliva collection with the infant swab when the infants were allowed to chew or suck on the swab for 30-60 seconds, followed by mopping up any pooled saliva left in the mouth or on the face. The samples were then recovered immediately by compression in a syringe, and the procedure was repeated to collect more saliva until a sufficient volume was reached. When the samples arrived at our testing laboratory for testosterone analysis, most of the volumes were around 1 mL, and there were no samples with insufficient volume.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)