Need Help?
Ask an expert
1. How to collect Salivary Cotinine
APPROVED SALIVARY COTININE COLLECTION METHODS
Salivary Cotinine Collection Protocol
Collection volume, general considerations, and basic guidelines to maximize salivary cotinine sample integrity. Use this analyte-specific collection protocol to plan you collection methodology and sampling schemes.

2. How to Assay for Salivary Cotinine
Send Saliva Samples to Salimetrics
Add to StudyEasy and accurate results from the most trusted Salivary Bioscience Laboratory.
All Lab ServicesOrder Code5150
Salivary Cotinine ELISA Kit
Add to Study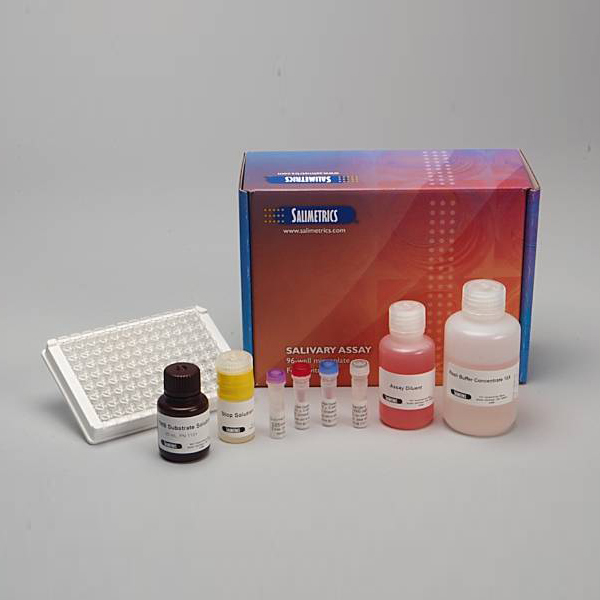
Salimetrics Assay #1-2002
Salimetrics Salivary Cotinine ELISA provides researchers with a sensitive method to objectively quantify differences in individual cotinine levels (nicotine exposure), using an easy to collect, non-invasive saliva-sample. Validated for clinical use, and highly correlated with serum levels, Salimetrics Cotinine assay’s high-sensitivity distinguishes between active smokers, non-smokers, and recent second-hand smoke exposure. Cotinine, the most common biomarker for nicotine exposure analysis, and is primarily measured in smoking cessation studies, e-cigarette and public health research, and to assess second-hand smoke exposure in children. Companies may also evaluate salivary cotinine levels for tobacco use relative to employment, health, and life-insurance purposes. Cotinine is a preferred biomarker to evaluate tobacco use, because it maintains a half-life of up to 16-hours, as opposed to nicotine’s half-life of just several hours. Read More...| Assay Protocol |
|---|
| Rev. 04.19.19
|
| Specifications | |
|---|---|
| Catalog#: | 1-2002 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 2.5 hrs |
| Sample Volume/Test: | 20 µL |
| Sensitivity: | 0.15 ng/mL |
| Assay Range: | 0.8 ng/mL - 200 ng/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 76 |
| Duplicate: | 38 |
| Target Analyte |
|---|
Technical Documentation
Assay Kit Overview
Intended Use
The Salimetrics Cotinine Enzyme Immunoassay Kit is a competitive immunoassay specifically designed and validated for the quantitative measurement of salivary Cotinine. This kit may be used to measure primary or secondhand exposure to nicotine. It is not intended for diagnostic use. It is intended only for research use in humans and some animals. Salimetrics has not validated this kit for serum or plasma samples, however a validated urine protocol is available upon request.
Introduction
The detection of exposure to tobacco smoke by measurement of Cotinine is the preferred method. Nicotine is not considered a valid marker of smoking status due to its relatively short half-life (approximately 2 hours). By contrast, Cotinine has an average half-life of 17 hours, and blood levels closely reflect the dose of nicotine absorbed from tobacco smoke. Saliva samples are easier to obtain over alternative biofluids, however, and saliva levels are highly correlated and used interchangeably with blood levels.
Many studies show that levels of Cotinine in saliva show large inter-individual differences. The sources of these differences include factors related to intrinsic and extrinsic predispositions that affect the physiology of nicotine metabolism, the dose of nicotine present in the cigarette (or alternative source), and health behaviors relevant to how nicotine is used (e.g., cigarette vent blocking, duration and frequency of puffs). There is a clear advantage to providing inexpensive, accurate, quantitative, and noninvasive means of validating nicotine use, measuring the immediate physiological consequences of individual differences and intra-individual change in user’s behaviors, and determining secondhand tobacco smoke exposure. Salimetrics has designed this research tool to provide biomedical researchers with a highly sensitive and reliable means to do so.
Salivary Cotinine Assay Principle
This is a competitive immunoassay kit. Cotinine in standards and samples compete with Cotinine conjugated to horseradish peroxidase for the antibody binding sites on a microtitre plate. After incubation, unbound components are washed away. Bound Cotinine Enzyme Conjugate is measured by the reaction of the horseradish peroxidase enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The amount of Cotinine Enzyme Conjugate detected is inversely proportional to the amount of Cotinine present in the sample.
Assay Comparison to LC-ESI/MS/MS
| (n=40) | Cotinine Results by LC-ESI/MS/MS | Cotinine Results by Salimetrics EIA |
|
LC-ES/MS/MS |
— | 0.90 (0.00) |
|
Salimetrics Cotinine EIA |
0.90 (0.00) | — |
|
Total Hrs. Exposed ^ |
0.39 (0.01) | 0.48 (0.00) |
Diagnostic Salivary Cotinine ELISA Kit (FDA, CE Mark)
Add to Study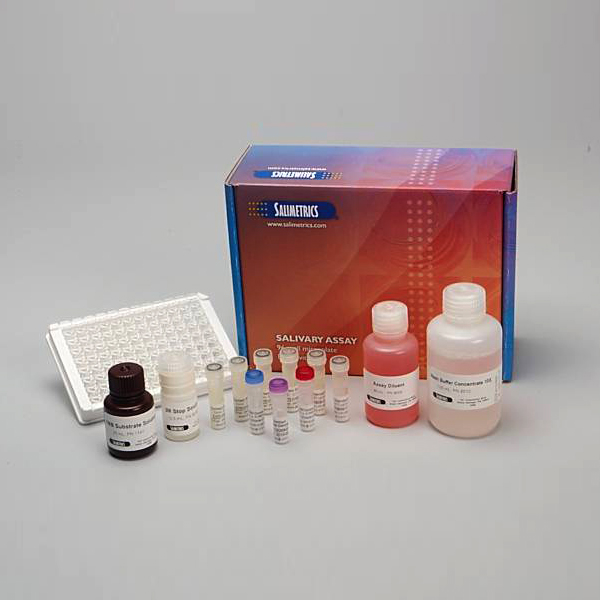
Salimetrics Assay #1-2112 (in vitro diagnostic use)
Salimetrics Salivary Cotinine ELISA provides researchers with a sensitive method to objectively quantify differences in individual cotinine levels (nicotine exposure), using an easy to collect, non-invasive saliva-sample. Validated for clinical use, and highly correlated with serum levels, Salimetrics Cotinine assay’s high-sensitivity distinguishes between active smokers, non-smokers, and recent second-hand smoke exposure. Cotinine, the most common biomarker for nicotine exposure analysis, and is primarily measured in smoking cessation studies, e-cigarette and public health research, and to assess second-hand smoke exposure in children. Companies may also evaluate salivary cotinine levels for tobacco use relative to employment, health, and life-insurance purposes. Cotinine is a preferred biomarker to evaluate tobacco use, because it maintains a half-life of up to 16-hours, as opposed to nicotine’s half-life of just several hours. Read More...| Assay Protocol |
|---|
| Rev. 01.04.21
|
| Specifications | |
|---|---|
| Catalog#: | 1-2112 |
| Regulatory Status: | 510(k) Exempt, CE Mark |
| Format: | 96-well plate |
| Assay Time: | ~ 2.5 hrs |
| Sample Volume/Test: | 20 µL |
| Sensitivity: | 0.15 ng/mL |
| Assay Range: | 0.8 ng/mL - 200 ng/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 76 |
| Duplicate: | 38 |
| Target Analyte |
|---|
Technical Documentation
3. Technical Summary
| Analyte Summary | |
|---|---|
| Analyte: | Cotinine |
| Aliases: | Biomarker of Nicotine Exposure |
| Serum-Saliva Correlation: | NA |
| *Optimum Collection Volume: | 75 μL |
| Assay Summary | |
|---|---|
| Methodology: | ELISA |
| Sensitivity: | 0.15 ng/mL |
| Assay Range: | 0.8 ng/mL - 200 ng/mL |
| Assay Type: | Quantitative |
| Salivary Cotinine Example Ranges* | ||||
|---|---|---|---|---|
| Group | Number | Mean (ng/mL) | Standard Deviation (ng/mL) | Range (ng/mL) |
| Adult Smokers | 21 | 206.33 | 123.47 | 47.87-586.39 |
| Non-Smokers | 10 | 0 | 0 | NA |
Background
When nicotine from tobacco smoke is taken into the lungs and enters the bloodstream, the principle metabolite produced in the liver is cotinine. Cotinine diffuses easily from blood into saliva, and salivary cotinine and blood levels are highly correlated. (1) Cotinine in saliva has a longer half-life than nicotine (more than 10 hours), and the literature has documented it to be a specific and sensitive marker for determining exposure to tobacco and nicotine, allowing for primary and second-hand exposure determination. (2,3,4)
References & Salivary Cotinine Research
- Benowitz, N.L. (1996). Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev, 18(2), 188-204.
- Dhar, P. (2004). Measuring tobacco smoke exposure: Quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J Pharm Biomed Anal, 35(1), 155- 68.
- Alterman, A.I., Gariti, P., Niedbala, R.S. (2002). Varying results for immunoassay screening kits for cotinine levels. Psychol Addict Behav, 16(3), 256-59.
- Van Vunakis, H., Tashkin, D.P., Rigas, B., et al. (1989). Relative sensitivity and specificity of salivary and serum cotinine in identifying tobacco-smoking status of self-reported non-smokers and smokers of tobacco and/or marijuana. Arch Environ Health, 44(1), 53-58.
- Benowitz, N.L., Hukkanen, J., & Jacob, P., 3rd (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol, 192, 29-60.
- Watts, R.R., Longone, J.J., Kinght, G.J., & Lewtas, J. (1990). Cotinine analytical workshop report: Consideration of analytical methods for determining cotinine in human body fluids as a measure of passive exposure to tobacco smoke. Environ Health Perspect, 84, 173-82.
- Roche, D., Callai, F., Reungoat, P., & Momas, I. (2001). Adaptation of an enzyme immunoassay to assess urinary cotinine in nonsmokers exposed to tobacco smoke. Clin Chem, 47(5), 950-52.
- Chard, T. (1990). An introduction to radioimmunoassay and related techniques (4th ed.). Amsterdam: Elsevier.
- Granger, D.A., Blair, C., Willoughby, M., Kivlighan, K.T., Hibel, L.C., Fortunato, C.K., Wiegand, L.E., & The Family Life Project Investigators (2007). Individual differences in salivary cortisol and alpha-amylase in mothers and infants: Relation to tobacco smoke exposure. Dev Psychobiol, 49(7), 692-701.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)