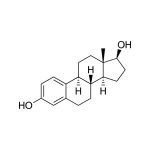
Diagnostic Salivary Estradiol ELISA Kit (FDA, CE Mark)
| Specifications |
|
Catalog#: |
1-4702 |
|
Regulatory Status: |
510(k) Exempt, CE Mark |
|
Format: |
96-well plate |
|
Assay Time: |
~ 3 hrs |
|
Sample Volume/Test: |
100 µL |
|
Sensitivity: |
0.1 pg/mL |
|
Assay Range: |
1 pg/mL - 32 pg/mL |
|
Storage Requirements: |
2-8°C |
| Tests Per Kit |
|
Singlet: |
76 |
|
Duplicate: |
38 |
References & Salivary Estradiol Research
-
- Abraham, G.E. (1975). The applications of steroid radioimmunoassay to gynecologic endocrinology. In: Taymor, M.L. and Green, T.H. (eds.): Progress in gynecology, Vol. 1, 111-144. New York: Grune and Stratton.
- Faiman, C., Winter, S.D., & Reyes, F.I. (1976). Patterns of gonadotropins and gonadal steroids throughout life. Clin Obstet Gynecol, 3(3), 467-483.
- Kirschner, M.A., Schneider, G., Ertel, N.H., Worton, E. (1982). Obesity, androgens, estrogens, and cancer risk. Cancer Res, 42 (8 suppl), 3281s-3285s.
- Labrie, F., Bélanger, A., Cusan, L., Candas, B. (1997). Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: Intracrinology. J Clin Endocrinol Metab, 82(8), 2403-9.
- Lipson, S.F., & Ellison, P.T. (1996). Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod, 11(10), 2090-96.
- Choe, J.K., Khan-Dawood, F.S., Dawood, M.Y. (1982). Progesterone and estradiol in saliva and plasma during the menstrual cycle. Am J Obstet Gynecol, 146, 557-62.
- Bao, A.-M., Liu, R.-Y., van Someren, E.J., et al. (2003). Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol, 148(2), 227-32.
- Chang, R.J., Plouffe Jr., L. Schaffer, K. Physiology of the menopause. In: Comprehensive management of menopause, Lorrain, J., Flouffe Jr., L., Ravnikar, V., Speroff, L., Watts, N., eds. New York: Springer, 1993.
- Reed, M.J., Lai, L.C., Owen, A.M., et al. (1990). Effect of treatment with 4-hydroxyandrostenedione on the peripheral conversion of androstenedione to estrone and in vitro tumor aromatase activity in postmenopausal women with breast cancer. Cancer Res, 50(1), 193-96.
- Shirtcliff, E.A., Dahl, R.E., Pollak, S.D. (2009). Pubertal development: Correspondence between hormonal and physical development. Child Dev, 80(2), 327-37.
-
- Simpson, E.R. (2000). Role of aromatase in sex steroid action. J Mol Endocrinol, 25(2), 149-56.
- Winters, S.J., Troen, P. (1986). Testosterone and estradiol are co-secreted episodically by the human testis. J Clin Invest, 78(4), 870-73.
- Nankin, H.R., Pinto, R., Fan, D.-F., Troen, P. (1975). Daytime titers of testosterone, LH, estrone, estradiol, and testosterone-binding protein: Acute effects of LH and LH-releasing hormone in men. J Clin Endocrinol Metab, 41(2), 271-81.
- Ouyang, P., Michos, E.D., Kara, R.H. (2006). Hormone replacement therapy and the cardiovascular system: Lessons learned and unanswered questions. J Am College Cardiol, 47(9), 1741-53.
- McCarthy, M.M. (2008). Estradiol and the developing brain. Physiol Rev, 88(1), 91-134.
- Balthazart, J., Cornil, C.A., Taziaux, M., et al. (2006). Rapid changes in production and behavioral action of estrogens. Neuroscience, 138(3), 783-91.
- Karpuzoglu, E., Ahmed, S.A. (2006). Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: Implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide, 15(3), 177-86.
- Colditz, G. A. 1998. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst, 90(11), 814-23.
- Lépine, J., Audet-Walsh, E., Grégoire, J., et al. (2010) Circulating estrogens in endometrial cancer cases and their relationship with tissular expression of key estrogen biosynthesis and metabolic pathways. J Clin Endocinol Metab, 95(6), 2689-98.
- Sasano, H., Harada, N. (1998). Intratumoral aromatase in human breast, endometrial, and ovarian malilgnancies. Endocr Rev, 19(5), 593-607.
- Vining, R.F., & McGinley, R.A. (1987). The measurement of hormones in saliva: Possibilities and pitfalls. J Steroid Biochem, 27(1-3), 81-94.
- Choe, J.K., Khan-Dawood, F.S., & Dawood, M.Y. (1983). Progesterone and estradiol in saliva and plasma during the menstrual cycle. Am J Obstet Gynecol, 147(5), 557-62.
- Shirtcliff, E.A., Granger, D.A., Schwartz, E.B., et al. (2000). Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Horm Behav, 38(2), 137-47.
- Ellison, P.T., Lipson, S.F. (1999). Salivary estradiol–A viable alternative? Fertil Steril, 72(5), 951-52.)
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)