Need Help?
Ask an expert
1. How to collect Salivary Estradiol
APPROVED SALIVARY ESTRADIOL COLLECTION METHODS
Salivary Estradiol Collection Protocol
Collection volume, general considerations, and basic guidelines to maximize salivary estradiol sample integrity. Use this analyte-specific collection protocol to plan you collection methodology and sampling schemes.

2. How to Assay for Salivary Estradiol
Send Saliva Samples to Salimetrics
Add to StudyEasy and accurate results from the Salimetrics CoreLab+ Laboratory.
Order Code5160
Salivary Estradiol ELISA Kit
Add to Study
Salimetrics Assay #1-3702
The Salimetrics Salivary Estradiol (17-beta estradiol, oestradiol) Enzyme Immunoassay Kit was specifically designed to standardize the detection of estradiol in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range that spans the expected estradiol levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This estradiol assay kit has also been formatted to minimize cross reactivity for related steroids.Estradiol (17β-estradiol, E2, 1,3,5(10)-estratriene-3, 17β-diol) is the most active naturally secreted estrogen. In men, estradiol originates in the testes and from extraglandular conversion of androgens. Circulating estradiol levels are relatively high at birth in both males and females, but decrease postnatally. Research concerning estradiol has focused predominantly on reproductive issues such as conception, ovulation, infertility, and menopause. Yet, estradiol affects a diversity of biological processes involved with pubertal and reproductive capacity, establishment and maintenance of pregnancy, infant care, coronary artery disease, immunocompetence, and cancer susceptibility. Estradiol is also believed to affect individual differences in cognitive and socioemotional processes, as well as psychopathology. Read More...| Assay Protocol |
|---|
| Rev. 01.30.26
|
| Specifications | |
|---|---|
| Catalog#: | 1-3702 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 100 µL |
| Sensitivity: | 0.1 pg/mL |
| Assay Range: | 1 pg/mL - 32 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 78 |
| Duplicate: | 39 |
| Target Analyte |
|---|
Technical Documentation
Assay Kit Overview
Intended Use
The Salimetrics 17β-Estradiol Enzyme Immunoassay Kit is a competitive immunoassay specifically designed and validated for the quantitative measurement of salivary Estradiol. It is not intended for diagnostic use. It is intended only for research use in humans and some animals. Salimetrics has not validated this kit for serum or plasma samples.
Introduction
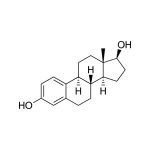
Estradiol (17β-Estradiol, E2, 1,3,5(10)-estratriene-3, 17β-diol), a steroid hormone, is produced primarily by the ovarian follicles from testosterone. Estradiol is the most active naturally secreted estrogen. In men, Estradiol originates in the testes and from extraglandular conversion of androgens. Circulating Estradiol levels are relatively high at birth in both males and females, but decrease postnatally. In prepubertal children and men, levels are non-cyclic and low. During puberty, there are gradual increases in Estradiol levels in both males and females. Interactions between luteinizing hormone (LH) and follicle-stimulating hormone (FSH) cause the release of Estradiol from the ovaries in premenopausal women. Estradiol secretion is low in postmenopausal women. Research concerning Estradiol has focused predominantly on reproductive issues such as conception, ovulation, infertility, and menopause. Yet, Estradiol affects a diversity of biological processes involved with reproductive capacity, establishment and maintenance of pregnancy, parenting, coronary artery disease, immunocompetence, cancer susceptibility, and neuroprotection. Estradiol is also believed to affect individual differences in cognitive and socioemotional processes as well as psychopathology. Estrogens have been measured by many immunoassay methods. Studies suggest that Estradiol can be accurately measured in saliva.
Salivary Estradiol Assay Principle
This is a competitive immunoassay kit. Estradiol in standards and samples compete with Estradiol conjugated to horseradish peroxidase for the antibody binding sites on a microtitre plate. After incubation, unbound components are washed away. Bound Estradiol Enzyme Conjugate is measured by the reaction of the horseradish peroxidase enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The amount of Estradiol Enzyme Conjugate detected is inversely proportional to the amount of Estradiol present in the sample.
Diagnostic Salivary Estradiol ELISA Kit (FDA, CE Mark)
Add to Study
Salimetrics Assay #1-4702 (in vitro diagnostic use)
The Salimetrics Salivary Estradiol (17-beta estradiol, oestradiol) Enzyme Immunoassay Kit was specifically designed to standardize the detection of estradiol in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range that spans the expected estradiol levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This estradiol assay kit has also been formatted to minimize cross reactivity for related steroids. Estradiol (17β-estradiol, E2, 1,3,5(10)-estratriene-3, 17β-diol) is the most active naturally secreted estrogen. In men, estradiol originates in the testes and from extraglandular conversion of androgens. Circulating estradiol levels are relatively high at birth in both males and females, but decrease postnatally. Research concerning estradiol has focused predominantly on reproductive issues such as conception, ovulation, infertility, and menopause. Yet, estradiol affects a diversity of biological processes involved with pubertal and reproductive capacity, establishment and maintenance of pregnancy, infant care, coronary artery disease, immunocompetence, and cancer susceptibility. Estradiol is also believed to affect individual differences in cognitive and socioemotional processes, as well as psychopathology. Read More...| Assay Protocol |
|---|
| Rev. 01.30.26
|
| Specifications | |
|---|---|
| Catalog#: | 1-4702 |
| Regulatory Status: | 510(k) Exempt, CE Mark |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 100 µL |
| Sensitivity: | 0.1 pg/mL |
| Assay Range: | 1 pg/mL - 32 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 78 |
| Duplicate: | 39 |
| Target Analyte |
|---|
Technical Documentation
3. Technical Summary
| Analyte Summary | |
|---|---|
| Analyte: | Estradiol |
| Aliases: | oestradiol, 17β-estradiol, E2 |
| Serum-Saliva Correlation: | 0.80 |
| Optimum Collection Volume: | 225 μL* |
| Assay Summary | |
|---|---|
| Methodology: | ELISA |
| Sensitivity: | 0.1 pg/mL |
| Assay Range: | 1 pg/mL - 32 pg/mL |
| Assay Type: | Quantitative |
Background
Estradiol is one of the three main estrogenic steroid hormones present in humans. It is the most active naturally secreted estrogen. (1) In menstruating women, estradiol is produced primarily by the ovarian follicles from testosterone, with additional amounts produced by extraglandular conversion of testosterone in peripheral tissues. (1,2,3,4) Concentrations peak mid-cycle, marking ovulation, followed by a rapid decline with a smaller secondary increase during the luteal phase. (5,6) In women of reproductive age, estradiol exhibits a diurnal rhythm where the peaks tend to occur in the early morning; the timing of the peaks is shifted later during the menstrual phase. There are also ultradian harmonics superimposed upon the basic diurnal rhythm. (7) In post-menstrual women, small amounts of estradiol continue to be made from estrone and testosterone in the peripheral tissues, but estrone, also produced peripherally, replaces it as the predominant form of estrogen. (8,9) In women, estradiol is responsible for the development of secondary sexual characteristics, enhancing breast development and affecting body shape, bones, joints, and fat deposition. (10) In men and pre-pubertal children, estradiol originates principally from extraglandular conversion of androgens; men also have small amounts produced in the testes. (11,12) In men there is no diurnal rhythm to estradiol production. (13) In addition to its role in sexual and reproductive functioning, estradiol also affects other parts of the body, including the cardio-vascular system, the brain, and the immune system. (14-17) Estradiol has also been studied for strong relationships with cancers of the breast, ovary, and uterine lining. (18-20) In the blood only 1 to 15% of estradiol is in its unbound or biologically active form. The remaining estradiol is bound to serum proteins. Unbound serum estradiol enters saliva via intracellular mechanisms, and in saliva the majority of estradiol remains unbound to protein. (21) The correlation between serum and saliva samples is high. (22-24)
References & Salivary Estradiol Research
-
- Abraham, G.E. (1975). The applications of steroid radioimmunoassay to gynecologic endocrinology. In: Taymor, M.L. and Green, T.H. (eds.): Progress in gynecology, Vol. 1, 111-144. New York: Grune and Stratton.
- Faiman, C., Winter, S.D., & Reyes, F.I. (1976). Patterns of gonadotropins and gonadal steroids throughout life. Clin Obstet Gynecol, 3(3), 467-483.
- Kirschner, M.A., Schneider, G., Ertel, N.H., Worton, E. (1982). Obesity, androgens, estrogens, and cancer risk. Cancer Res, 42 (8 suppl), 3281s-3285s.
- Labrie, F., Bélanger, A., Cusan, L., Candas, B. (1997). Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: Intracrinology. J Clin Endocrinol Metab, 82(8), 2403-9.
- Lipson, S.F., & Ellison, P.T. (1996). Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod, 11(10), 2090-96.
- Choe, J.K., Khan-Dawood, F.S., Dawood, M.Y. (1982). Progesterone and estradiol in saliva and plasma during the menstrual cycle. Am J Obstet Gynecol, 146, 557-62.
- Bao, A.-M., Liu, R.-Y., van Someren, E.J., et al. (2003). Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol, 148(2), 227-32.
- Chang, R.J., Plouffe Jr., L. Schaffer, K. Physiology of the menopause. In: Comprehensive management of menopause, Lorrain, J., Flouffe Jr., L., Ravnikar, V., Speroff, L., Watts, N., eds. New York: Springer, 1993.
- Reed, M.J., Lai, L.C., Owen, A.M., et al. (1990). Effect of treatment with 4-hydroxyandrostenedione on the peripheral conversion of androstenedione to estrone and in vitro tumor aromatase activity in postmenopausal women with breast cancer. Cancer Res, 50(1), 193-96.
- Shirtcliff, E.A., Dahl, R.E., Pollak, S.D. (2009). Pubertal development: Correspondence between hormonal and physical development. Child Dev, 80(2), 327-37.
-
- Simpson, E.R. (2000). Role of aromatase in sex steroid action. J Mol Endocrinol, 25(2), 149-56.
- Winters, S.J., Troen, P. (1986). Testosterone and estradiol are co-secreted episodically by the human testis. J Clin Invest, 78(4), 870-73.
- Nankin, H.R., Pinto, R., Fan, D.-F., Troen, P. (1975). Daytime titers of testosterone, LH, estrone, estradiol, and testosterone-binding protein: Acute effects of LH and LH-releasing hormone in men. J Clin Endocrinol Metab, 41(2), 271-81.
- Ouyang, P., Michos, E.D., Kara, R.H. (2006). Hormone replacement therapy and the cardiovascular system: Lessons learned and unanswered questions. J Am College Cardiol, 47(9), 1741-53.
- McCarthy, M.M. (2008). Estradiol and the developing brain. Physiol Rev, 88(1), 91-134.
- Balthazart, J., Cornil, C.A., Taziaux, M., et al. (2006). Rapid changes in production and behavioral action of estrogens. Neuroscience, 138(3), 783-91.
- Karpuzoglu, E., Ahmed, S.A. (2006). Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: Implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide, 15(3), 177-86.
- Colditz, G. A. 1998. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst, 90(11), 814-23.
- Lépine, J., Audet-Walsh, E., Grégoire, J., et al. (2010) Circulating estrogens in endometrial cancer cases and their relationship with tissular expression of key estrogen biosynthesis and metabolic pathways. J Clin Endocinol Metab, 95(6), 2689-98.
- Sasano, H., Harada, N. (1998). Intratumoral aromatase in human breast, endometrial, and ovarian malilgnancies. Endocr Rev, 19(5), 593-607.
- Vining, R.F., & McGinley, R.A. (1987). The measurement of hormones in saliva: Possibilities and pitfalls. J Steroid Biochem, 27(1-3), 81-94.
- Choe, J.K., Khan-Dawood, F.S., & Dawood, M.Y. (1983). Progesterone and estradiol in saliva and plasma during the menstrual cycle. Am J Obstet Gynecol, 147(5), 557-62.
- Shirtcliff, E.A., Granger, D.A., Schwartz, E.B., et al. (2000). Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Horm Behav, 38(2), 137-47.
- Ellison, P.T., Lipson, S.F. (1999). Salivary estradiol–A viable alternative? Fertil Steril, 72(5), 951-52.)
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)